Two Isotopes With the Same Number of Protons
In the same column of the periodic table. Isotopes are atoms of an element with the normal number of protons and electrons but different numbers of.

Question Video Recognize That Only Nuclei With Different Numbers Of Neutral Particles Can Be Different Isotopes Nagwa
So one would be hydrogen to another would be lithium six and another one would be bore on 10 then two isotopes with the same number of protons.

. In contrast the proton numbers for which there are no stable isotopes are 43 61 and 83 or more. An isotope has same atomic number but different mass number. Two nuclides are isotones if they have the same neutron number N but different proton number Z.
The same number of neutrons and electrons but different numbers of protons. 1 H 1 Protium 2 H 1 Deutetrium 3 H 1 Tritium are. Atoms with the same number of protons but with different numbers of neutrons.
The number of protons in a nucleus determines the elements atomic number on the Periodic Table. Isotopes are generally named by their mass numbers such as Cobalt-60. Two different isotopes of the same element have a.
An acid salt Answer Correct option is C an isotope An atom with the same number of protons and same number of electrons but a different number of neutrons is an isotope. Isotopes of the same element. Carbon 12 which has 6 neutrons plus 6 protons equals 12 carbon 13 which has 7 neutrons and carbon 14 which has 8 neutrons.
Two atoms are isotopes of the same element if they have the same number of protons and therefore also the same number of electrons BUT different numbers of neutrons. When working with Isotopes of the same element they have a matching number of protons the atomic number and electrons. The same number of protons and neutrons but different numbers of electrons.
Not ions that have the same number of protons are considered to belong to the same element but by adding different numbers of neutrons multiple isotopes. Add your answer and earn points. Two atoms with the same number of protons but a different number of neutrons are 2 points 1.
For example Carbon has three isotopes carbon-14 carbon-13 and carbon-12. Answer verified by Toppr Upvote 0. For example carbon has six protons and is atomic number 6.
Based on the definitions of atomic mass and atomic number provided above we may deduce that isotopes are elements that have the same atomic number but a different mass number. There are several different types of isotope notation. 35 - 17 18 neutrons.
Isotopes are the different variants of a single chemical element that possess the same number of protons and electrons but a different number of neutrons. 1 See answer Add answer 5 pts anamfarrant is waiting for your help. A Z of neutrons Section 24 Isotopes of Elements Symbols and names for isotopes include the element name or symbol and the mass number.
13 6C 613C carbon-13 or CC-13. The same number of protons neutrons and electrons. Dec 2 2016 Neutrons.
Two atoms with the same atomic number but different mass numbers same number of protons different number of neutrons are called isotopes or. Anumber of protonsnumber of neutronsAnumber of protonsnumber of neutrons. Isotopes have the same atomic number and are the same element but have different mass numbers.
Up to 24 cash back Atoms With The Same Number Of Protons But Different Numbers Of Neutrons In The Nucleus Of An Atom. Enter the appropriate symbol for an isotope. Atoms of the same element containing the same number of protons but different numbers of neutrons are known as isotopes.
M is the chemical symbol for the element. For example 1735 C l and 1737 C l are two isotopes of chlorine. Next to each other on the periodic table.
The same number of protons and electrons but different numbers of neutrons. Or in other words elements that have the same number of protons but different numbers of neutrons are called Isotopes. For example boron-12 and carbon-13 nuclei both contain 7 neutrons and so are isotones.
In other words we can say that atoms have the same atomic number but different mass numbers are called isotopes. Isotopes are often represented using the symbol. Two terms we use to identify nuclides isotopes are atomic number and mass number.
Ions of the same element. Isotopes of any given element all contain the same number of protons so they have the same atomic number for example the atomic number of helium is always 2. Any two neutrally charged atoms Ie.
Carbon occurs naturally in three isotopes. But theyd have a different number of neutrons. For example a carbon isotope that has an atomic number of 6 and a mass number of 13 could be symbolized in any of the following ways.
Two atoms with the same number of protons but Two atoms with the same number of protons but different numbers of neutrons are isotopes of the same element. 1 Answer Dee Ernest Z. It would be something like hydrogen one and hydrogen to have the same number of protons that needs to be the same element.
If this is the case than their mass number ends up being double their atomic number.

Isotopes Atoms Of The Same Element That Different Mass Numbers Physical Chemistry Atom Mass Number

Protons Neutrons Electrons Isotopes Average Mass Number Atomic Structure Atoms Vs Ions Youtube

Simplychemistry C1 1 2 Proton Number Mass Number Ions Isotopes
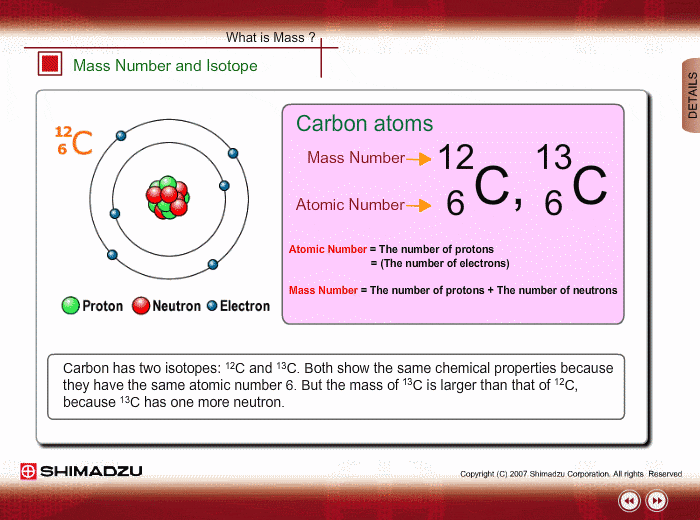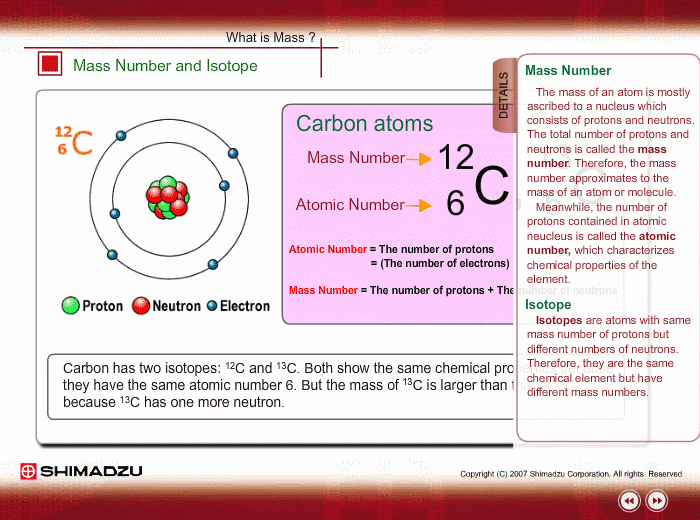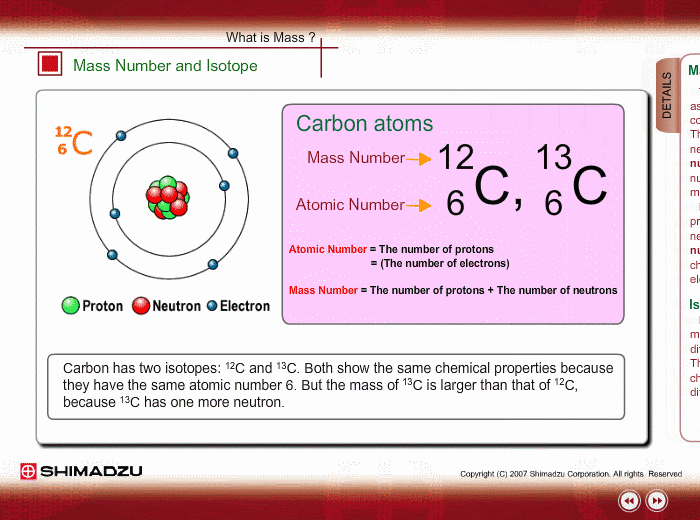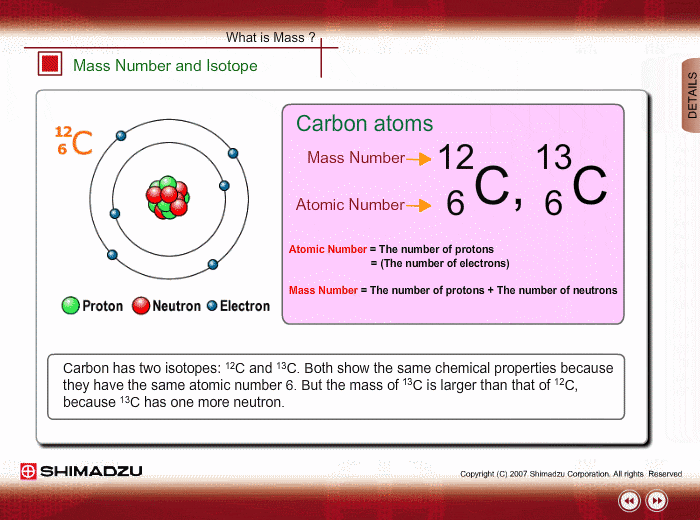
Mass Number And Isotope Shimadzu Shimadzu Corporation

Isotope Basics Nidc National Isotope Development Center

Protons Neutrons Electrons Isotopes Average Mass Number Atomic Structure Atoms Vs Ions Youtube

Lesson Explainer Isotopes Nagwa

Atomic Number Mass Number And Isotopes Video Khan Academy
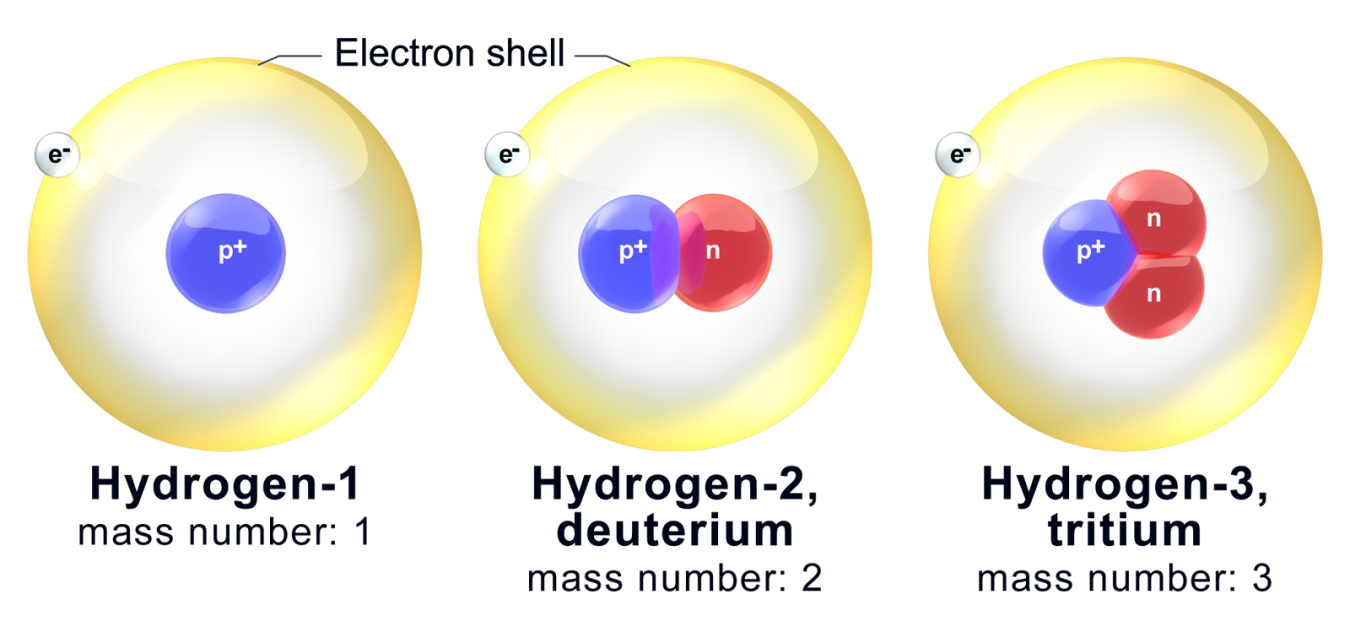
Doe Explains Isotopes Department Of Energy
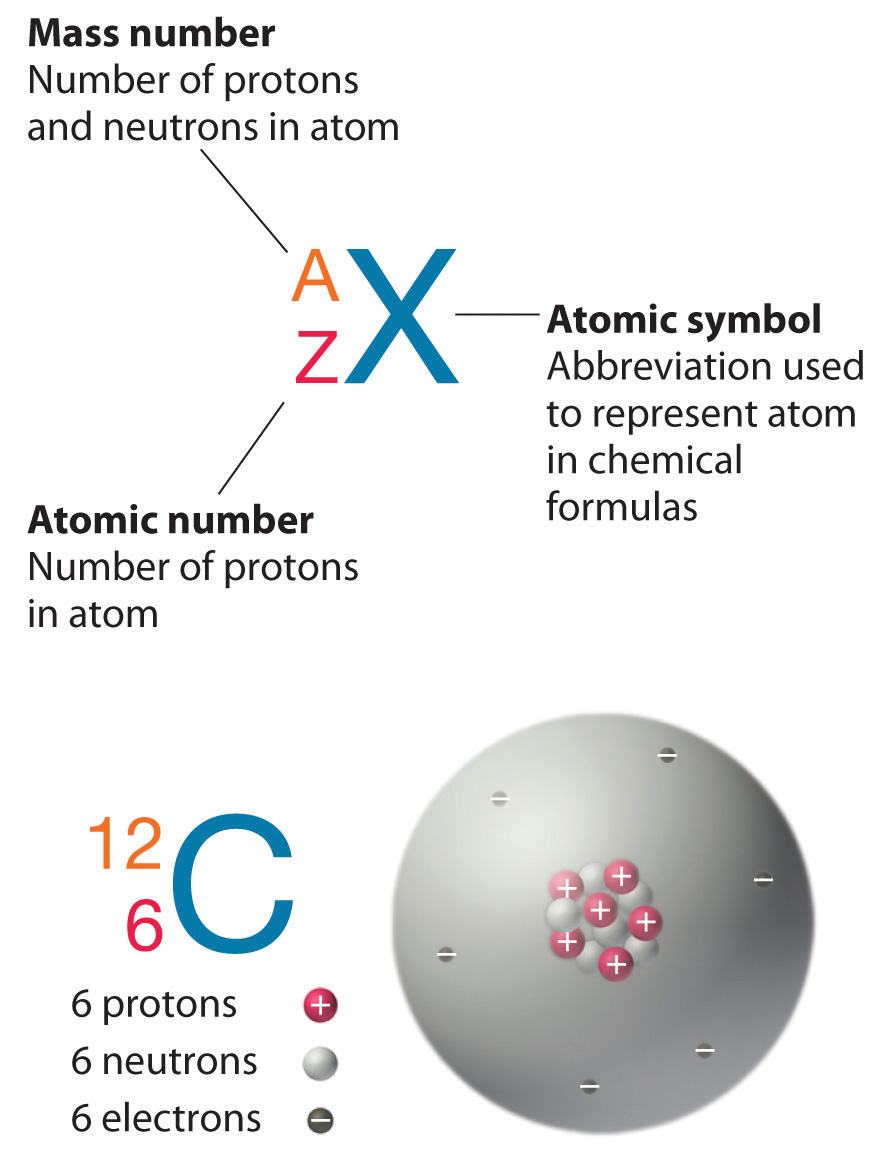
3 3 Isotopes Chemistry Libretexts
How Many Protons Are There In The C 12 Isotope How About The Number Of Neutrons Quora

Lesson Explainer Isotopes Nagwa
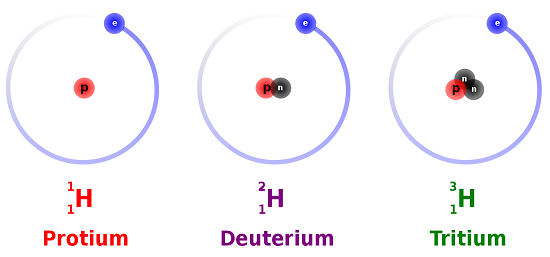
5 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts

Fundamentals Of Gc Ms Mass Number And Isotope Shimadzu

Iv Isotopes 2 Or More Atoms Of The Same Element Having The Same Number Of Protons But Different Numbers Of Neutrons Atomic Theory Neutrons Atom


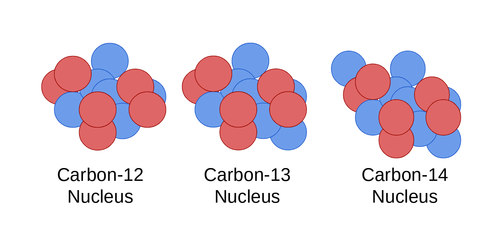
Comments
Post a Comment